 |
||||||||||
What is Orbital ?
3.1 Orbital based on Classical Mechanics
Firstly let us discuss the moon rotating around the earth in order to understand "orbital". In this case, the gravitational pulls between the earth and the moon is defined as follows.
![]()
Therefore the law of conservation of angular moment is proved as follows.
![]()
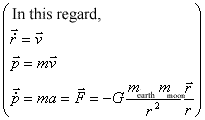
Therefore the angular moment does not depend on time and keeps constant. And it has same quantity and direction in any time. Moreover the rotational movement is performed in a plane and the angular moment determines the orbital.
3.2 Orbital based on Quantum Mechanics
When we focus on an atom, an electron is rotating around nucleus of the atom. So let us discuss Schrodinger equation in case of hydrigen. This picture is rotational movement of the moon rotating around the earth on the quantum mechanics.

Here ![]() .
. ![]() is spherical harmonics. This function determine the shape of the orbital. And we can express the following fomula.
is spherical harmonics. This function determine the shape of the orbital. And we can express the following fomula.
![]()
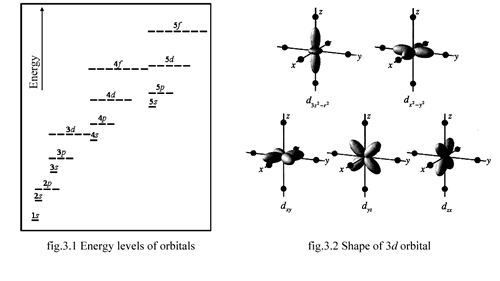
n determine the energy of the orbital. As we focus on the energy levels of each element. The more the quantitiy of n is, the more the energy level is [fig.3.1]. The shape of orbital depends on azimuthal quantum number: l and magnetic quantum number: m. For example the shape of 3d orbital depends on the magnetic quantum number [fig.3.2].
3.3 Orbital Degrees of Freedom
Let us introduce Perovskite-type transition metal oxides as the introduction of degrees of freedom in orbital.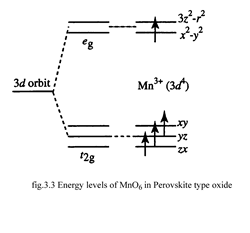
In the unitcell of the Perovskite-type transition metal oxide, one transition metal ion (M) is coordinated by the oxygen ions. And they forms MO6. As the oxygen ions make the crystal field lower, the energy level is splitted into 2 groups, t2g orbital (xy, yz, zx) and eg orbital (3z2-y2, x2-y2). Following Hund rule, the electron fills in lower energy orbital [fig.3.3]. In case of Mn3+ ion, electrical state of 3d orbital is (t2g)3(eg)1, it has the degrees of freedom on eg orbital, whether an electron fills in 3z2-y2 orbital or x2-y2 orbital. This is the orbital degrees of freedom.