 |
||||||||||
Copper Oxide Superconductor
In 1986, J. Bednorz and K. Müller reported copper-oxide superconductor in "Possible High Tc Superconductivity in the Ba-La-Cu-O System". Now a lot of 100 K grade copper-oxide superconductors are discovered. The name of "a copper oxide" comes from CuO2 plane made up of copper and oxygen in the crystal structure.Fig.2 indicates crystal structure in "La2-xBaxCuO4", the first copper-oxide superconductor.This structure is called K2NiF4 type, and perovskite structure and rock salt type structure are stacked alternately. An arrow shows CuO2 plane in this figure. The CuO2 plane plays an important role in superconductivity in copper-oxide superconductors.

Key factors in superconductivity mechanism ~ electronic state and carrier dope in CuO2 plane ~
Copper oxide superconductor has high Tc. but the mechanism has not been understood for 20 years. There are some common points. The mother materials are anti-ferromagnetism insulators which have CuO2 plane, and realize superconductivity by carrier dope. Here, let us introduce the most famous material, "La2CuO4" which is mother material of the first copper-oxide superconductor La2-xBaxCuO4.
Electronic
state of CuO2 plane (1) ~ Mott insulator and anti-ferromagnetism
order correlation ~
Copper-oxide
superconductor has two-dimensional plane (CuO2 plane) made up of
copper and oxygen in the crystal structure. This CuO2 plane greatly contributes
to the superconductivity. Mother material consists of following ions, (La3+)2(Cu2+)(O2-)4.
Focusing on the crystal structure, CuO2 plane and (LaO)2 layer made up of La3+ and O2- piled up in turn. CuO2 plane is totally -2
values. (LaO)2 layer is +2 values. Therefore the system keeps neutral.
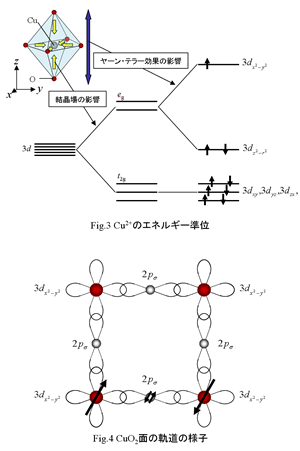
In
the CuO2 plane, electronic states in Cu2+ and O2- are (3d)9 and (2p)6 respectively, so 3dx2-y2 orbital in Cu lacks one electrons. Fig.3 shows energy level in Cu2+ in La2CuO4. 3d orbital of Cu2+ is
degenerate to five fold, so five orbits are called 3dxy (x-y plane), 3dyz (y-z plane), 3dzx (z-x plane), 3dx2-y2 (x axis and y axis direction), 3dz2-r2 (z axis
direction). The five
orbital are the same energy level, but they are affected by eight
oxygen around Cu, and 3dx2-y2 and 3dz2-r2 become higher energy level because of of oxygen position. Therefore, 3d orbital which degenerates
to five fold are divided to two energy level in t2g orbital
(3dx2-y2, 3dz2-r2)
and eg orbital (3dxy, 3dyz,
3dzx). We call it "crystal field". Furthermore,
in the case of La2CuO4, CuO6 octahedron expands to z axis direction (because this structure is energetically
stable), so 3dx2-y2 influenced more than 3dz2-r2. This is why energy level of
3dx2-y2 is highest. (dividing energy level caused by distorted ochtahedron is called "Jahn-Teller effect".) As a result,
in the case of electronic condition in Cu2+, only one electron exists
in 3dx2-y2. We show the state of orbital in
CuO2 plane in Fig.4, 3dx2-y2 orbit
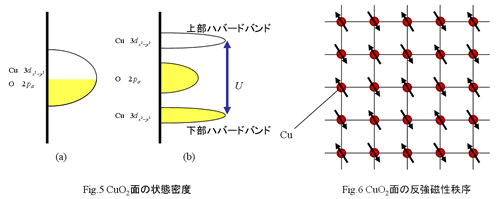
of Cu and 2p orbit of O forms hybridized orbital. The electronic state in CuO2 plane (3d)9 and half filled state in 3dx2-y2 (Fig.5(a)), so it should show
metallic property. But it has no conductivity, and mother maternal behaves as
an insulator. This comes from strong correlation between electrons. (The
system is called "strong correlation electronic system".) Under the conduction, an
electron of 3dx2-y2 must jump between Cu
sites. However
U, strong Coulomb interaction between electrons, prevents it when there
are two electrons. In other words, when
two electrons occupy one Cu site, it needs the extra energy U. Therefore conduction electron is suppressed and
localized in each Cu site. Therefire the band state in CuO2 plane is
Fig.5(b). Thanks to the
effect of big electronic correlation, 3d band in Cu is divided to lower
Hubbard band. That is occupied by electron and upper Hubbard band is empty.
Then the gap (Mott-Hubbard gap) is formed between these bands. Therefore,
mother material in the copper oxide superconductor is an
insulator. The insulator originated from
strong electronic correlation is called "Mott
insulator". As a result, an electron with the spin of S=1/2 and one hole
localize in Cu sites, so Cu ion becomes a magnetic ion. That is why
the mother material shows anti-ferromagnetiv ordering. In
mother material, the spin of S = 1/2 on a Cu site orders antiferromagnetically.
This is caused by spin in a Cu site and two electrons in the O site. An arrow in Fig.4 expresses a
spin of an electron in each site. As
electrons are occupied entirely in the O site, there are two spins. Thanks to
correlation of anti-ferromagnetism order in a copper oxide, spins of a Cu sites
between the neighbors interacts through a spin of this O site. This interaction is called "super exchange interaction".
Electronic
state of CuO2 plane (2) ~carrier dope and superconductivity expression~
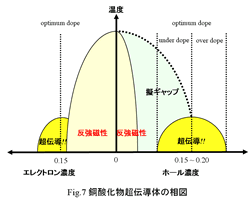
Big characteristic of a
copper oxide superconductor is that property of system changes into
anti-ferromagnetism ¨ superconductivity ¨ metal in depending on the carrier
density. Here, a career is a hall or an electron introduced into CuO2 plane. (It is substitution amount of Ln-214 system: x.) Fig.7 shows phase
diagram for career concentration x of copper oxide superconductor. A right
direction of a cross axle shows hall concentration x (La2-xSrxCuO4,
YBa2Cu3O6+Â, Bi2Sr2CaCu2O8+Â etcc). A left direction of a cross axle shows electron concentration x (Nd2-xCexCuO4 etcc). In
the domain where the hall density is small, doped material is
anti-ferromagnetic insulator by strong property of mother material. But, as the
hall density increases, it shows superconductivity. As increasing carrier
concentration, there is the domain where Tc increases (underdoping region) at
superconductivity phase. And, it is in the best Tc with a certain dope (optimal
dope). Then, on the contrary, it become domain that Tc decreases (overdoping
region). Most suitable dope amount x is around 0.15-0.20. While dope amount
increasing more, it do not show superconductivity, and becomes domain that a
metalic property is strong. (In the case of electron doping,
anti-ferromagnetism leniently decreases rather than in the case of hall doping,
and a superconductivity phase appears at the same time as decreasing
anti-ferromagnetism.) In addition, with a copper oxide superconductor, there is
a state such as superconductivity gap structure called "a pseudo gap" in normal state in T > Tc. Next, let's look at a
band state when superconductivity develops. A model to use here is the model
that is supported well when an electronic state of a copper oxide expressed. (It
is influential until now, and a decisive thing is not proposed in a copper
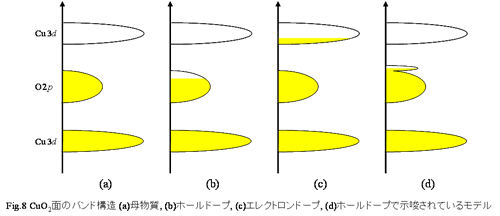
oxide superconductor.) Superconductivity phase appears, so it is expected that a band state of mother
material originally be motto insulator changed in a metal band state. As had
described first, a band of CuO2 plane in mother material seems to be
Fig.8 (a).At first let's think
about superconductor La2-xBaxCuO4 by hall doping. Electric charges of (LaO)2 layer decrease from +2
values by substituting La3+ for Ba2+, so an electric
charge of CuO2 plane which kept -2 values must change into a
direction of -1 value from -2 in order to keep an electric charge of the whole
system neutral in La2-xBaxCuO4.
In other words, an electron in a CuO2 plane must be removed from
there (put a hall into CuO2 plane). As a result, it is thought that
band structure of CuO2 plane which was originally an insulator band
state (Fig.8(a)) changes into a metallic band state like Fig.8(b) by removing
of electron (by injecting of hall).
On the other hand, in superconductor Nd2-xCexCuO4 by electron dope, because Nd3+ are substituted Ce4+ for,
electric charges of (NdO)2 layer increase from +2 values. Therefore
an electric charge of CuO2 plane changes from -2 values in a
direction of -3 values, that is, more electrons are injected into CuO2 plane. As a result, it is thought that the band structure becomes a condition
that electron entered in upper Hubbard band of 3d in Cu like Fig.8(c).
Such a band model thought about in a copper oxide superconductor, from being
thought in explanation of a semiconductor well, imitates semiconductor, and so
a superconductor by hall doping is called "a p type
superconductor" and a superconductor by electron doping is called "an n type superconductor".
In this way, both superconductors of p type and n type can be explained, so it is thought that this model is
influential. However, in the hall doping type that a single crystal is easily
grown in comparison with electron doping type, it is said that Fig.8 (d) which
is slightly different from Fig.8 (b) is convincing.As for this model, a new
state (impurity level with a semiconductor) is formed on a little above 2p band in O by hall doping, and with that, it behaves like metal.In addition, why can n
type and p type be really explained (to have a gap in Tc) by
the same model, in present conditions, room for various arguments is left. For
this mechanism elucidation, a new experiment result and discovery of a new
material are expected in future.
How to search new superconductors !? ~Characteristic of a copper oxide superconductor~
In the present when many copper oxide superconductors were discovered, information that leads to solution of superconductivity mechanism was prepared, but, on the other hand, the characteristic should become an indicator of a new superconductor search have been understood. "A general idea of block layer" showed in Fig.9 is one of them. crystal structure of a copper oxide superconductor is shown in Fig.9. The other atoms layer puts CuO2 plane, and CuO2 plane is alone. In La2-xSrxCuO4 of Fig.10, [(La,Sr)2O2] layer serves to isolate CuO2 plane. Such atom layer is called "the block layer". In other words a copper oxide takes the structure that CuO2 plane and the block layer piled up in turn. I described it in the first, the block layer plays a role in supplying of carrier for superconductivity expression whereas CuO2 plane plays a role in superconductivity. as a one characteristic in the copper oxides, without disturbing order of CuO2 plane(band structure etc), by adjustment of electric charge balance of the block layer, injecting of carrier for Superconductivity expression can was done. In this time, In La2-xSrxCuO4, I express that CuO2 plane is [CuO2]-2 and that the block layer is [(LaO)2]+2, and by doing Sr I can express that the block layer is [(La,Sr)2O2]+2-p, CuO2 plane is [CuO2]-2+p. This p becomes the carrier concentration. (p > 0 is correspond to hole doping, and p < 0 is correspond to electron doping.) In addition, an electric charge of the block layer certainly becomes an average of +2 values in mother material when I thought that the block layer is a unit lattice. (In Y-123, the block layer is Y3+ and [CuO chain]1+. However, when I thought in the whole unit lattice, the block layer becomes an average of +2.)
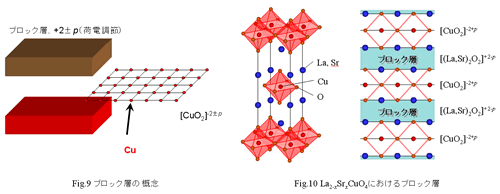
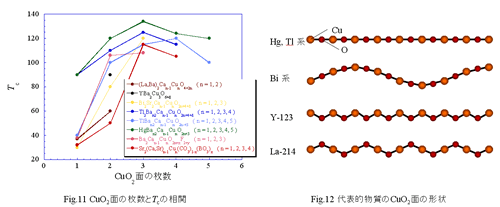
In this way I can express a copper oxide superconductor entirely by rearranging CuO2 plane and the block layer like a puzzle. Therefore, when I develop a new copper oxide superconductor I can do it mainly by devising composition of the block layer. Some interesting laws learned by experience are proposed by a lot of discovered copper oxide superconductors. One is relation between the number of sheets of CuO2 plane and Tc. Tc is 40 K in La2-xSrxCuO4 having one piece of CuO2 plane in a unit lattice. Tc is 90 K in YBa2Cu3O6+d having two pieces in a unit lattice. Tc is 134 K in HgBa2Ca2Cu3O8+Â having three pieces in a unit lattice. Tc tends to become surely high in much number of sheets of CuO2 plane. Then how is CuO2 plane in a material piling up endlessly? An answer is that it does not show even superconductivity. After all I can seem to say that the CuO2 plane that stood alone separated with the block layer is necessary. I show a value of Tc for the number of sheets of CuO2 side in a unit lattice in Fig.11 every each representative material. As watching this, we understand that Tc tends to become highest at three pieces of CuO2 plane. If number of CuO2 sides which stood alone is many, it seems to be disadvantage to develop high Tc adversely. In addition, it is also said shape of CuO2 plane is related to Tc. Shape of CuO2 plane of various copper oxide superconductors is shown in Fig.12. Though CuO2 plane of Hg, Tl system having high Tc in copper oxide superconductors is flat, those of La-214 system are uneven. As watching this, CuO2 plane is close in evenly seems to tend to expect high Tc. After discovery of La2-xBaxCuO4, a copper oxide superconductor nearly 200 kinds is reported. However, in the present when there are various laws learned by experience besides, you may discover a new superconductor having more high Tc by considering the thing which I introduced here.
Unique crystal structure of a copper oxide superconductor!!
In copper oxide superconductor, there is the common point that all composition shows superconductivity has CuO2 plane. However, it takes unique structure by the number of sheets of CuO2 plane in unit lattice (unit cell). Here, I introduce a main material among the crystal structure that there are dozens of kinds.
Ln-214
(having one piece of CuO2 plane) system
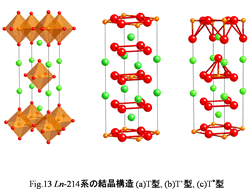
Crystal structure of three kinds of copper oxides is
shown in Fig.13. A
characteristic of these materials is having one piece of CuO2 plane
in a unit lattice. These
are usually called (a) T, (b) T', (c) T* type structure. At first, first copper oxide
superconductor La2-xBaxCuO4 is
equivalent to this T type (a). In T type, Cu and oxygen make octahedron (CuO6 octahedron), and CuO2 plane does the form that these CuO6 octahedron affected aside. Next, in Tf type (b), Nd2-xCexCuO4 showing superconductivity by electron doping takes this structure. In T' type, differently from T
type there is not oxygen (top oxygen) of a top part of CuO6 octahedron. On
this account CuO2 plane of T' type becomes a square. In last, T* type (c)
takes the structure that T type and T' type were combined. Therefore top oxygen occupied
only one side of CuO2 plane and forms CuO2 plane of a
pyramid type. (Nd,Ce)2-xSrxCuO4 shows superconductivity by this structure. A big characteristic in a superconductor of this
system is a point to control carrier by substituting it for one part of
element. In
the material which I introduced here, superconductivity develops by substituting Ln3+ for Ba2+ or Ce3+ partly.
Y-Ba-Cu-O
system
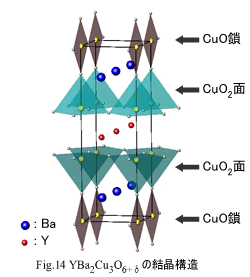
YBa2Cu3O6+Â (Y-123) to have recorded Tc of 90 K grade more than liquid nitrogen temperature for the first time are
showed in Fig.14. What carrier doping by Sr doping than Ba doping appeared high Tc and Tc having risen when pressure is
impressed in La2-xBaxCuO4 lead
to discover of Y-123. In other words it is an idea that an ion radius
which is smaller makes high Tc. As a result, Y-123 realized
high Tc more than 2 times of La2-xSrxCuO4.In
this system, materials have two pieces of CuO2 plane in a unit
lattice. Whereas
La2-xBaxCuO4 had CuO2 plane of octahedron, in Y-Ba-Cu-O system, octahedron is divided into up and
down, and CuO2 plane of top and bottom prevent connecting directly through top oxygen by Y
entering between it. Therefore
two pieces of CuO2 plane takes a pyramid type. In addition, a big
characteristic of crystal structure in this system is existence of a CuO chain
that connects a top of a pyramid. This chain site just was formed because oxygen
of an aspect direction of CuO2 plane suffers a loss. (If this part were CuO2 plane, three perovskites connects through top oxygen.) At this point, it can be said
that Y-Ba-Cu-O system has the most unique crystal structure in a copper oxide. A CuO chain gives big influence for
superconductivity expression of Y-Ba-Cu-O system. Though in Ln-214 system,
superconductivity develops by substituting the other element for a Ln site, in
this system, superconductivity develops by an oxygen ion entering in a chain
site. (Mother material of Y-123 system becomes YBa2Cu3O6 of the state that all the oxygen of a chain site fell out.) In superconductor
of Y-Ba-Cu-O system, there is YBa2Cu4O8(Y-124)
that a chain site repeated double, and Y2Ba4Cu7O14(Y-247)
which repeated Y-123 and Y-124 structure every one period other than Y-123. In addition, in the system that
replaced these Y sites with Ln superconductivity also develops.
Bi, Tl, Pb, Hg system
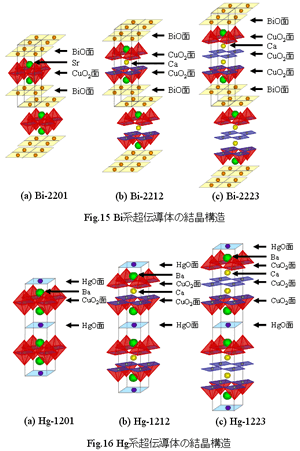
There are a lot of superconductors of Tc = 100 K grade in these system. A big characteristic is that numbers of CuO2 side in a unit cell increases, and Tc becomes high therewith by a Ca ion entering between CuO2 sides. In addition, in this system, oxygen supplies the carrier which is necessary for superconductivity expression.Fig.15 shows an example of a Bi system superconductor. (a) shows Bi2Sr2CuO6(Bi-2201), (b) shows Bi2Sr2CaCu2O8(Bi-2212), and (c) shows Bi2Sr2Ca2Cu3O10(Bi-2223) in turn. As watching Fig.15, we understand that none of the number of sheets of CuO2 plane increases when one Ca increases. The first report of the Bi system superconductor showed here was Bi-2201. What it is a superconductor of 30 K grade was reported later, but because the Tc at the time of discovery was 10 K degree, it was not attracting attention very much.However, several months later from discovery of Bi-2201, Bi2Sr2CaCu2O8(Bi-2212) and Bi2Sr2Ca2Cu3O10(Bi-2223) which added Ca to this were superconductors of 95 K, 110 K each was discovered.( There is the anecdote of very regret as a sign of Bi-2212 and Bi-2223 was seen by the resistance measurement in the laboratory where Bi-2201 was discovered.) Bi-2223 is first superconductor which recorded Tc more than 100K in a copper oxide superconductor. An important thing is that Ca and Sr have separate sites within crystal structure here. At that time, in searching a new superconductor, a normal way of thinking is that a big change does not arise by substituting for a similar element in chemical property. Sr and Ca are elements belonging to an alkaline-earth metal together. However, Sr is different from Ca in ion radius, so ion radius of Sr is 1.18 [đ], it of Ca is 1.02 [đ]. Though this difference had the same chemistry property, it leads to the result that could exist within crystal structure at the same time. After discovery of this Bi system, a superconductor having high Tc was discovered in Tl system by a search from a similar point of view.Now although a Hg system superconductor having a record of the best Tc is slightly different in crystal structure, but it has a similar characteristic that the number of CuO2 plane increase because a Ca ion enters it. Fig.16 shows crystal structure of a Hg system superconductor, (a) shows HgBa2CuO4+Â (Hg-1201), (b) shows HgBa2CaCu2O6+Â (Hg-1212), (c) shows HgBa2Ca2Cu3O8+Â (Hg-1223) sequentially. Tc is 90 K, 120 K, 134 K respectively. In this Hg system, Tc = 164 K of Hg-1223 under high pressure is the current best Tc. Same as Bi system, there is a material taking the crystal structure showed in Fig.16 in Tl system, Pb system, and it is reported as a superconductor to show high Tc together.
[1] J. G. Bednorz and K. A. Müller, Z. Phys. B64 (1986) 189
[2] BKBO
[3] S. Uchida, H. Takagi K. Kitazawa and S. Tanaka, Jpn. J. Appl. Phys. 26 (1987) L1
[4] M. K. Wu, J. R. Ashburn, C. J. Thorng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang and C. W. Chu, Phys. Rev. Lett. 58 (1987) 908
[5] H. Maeda, Y. Tanaka, M. Fukutomi and T. Asano, Jpn. J. Appl. Phys. 27 (1988) L209
[6] Y. Tokura, H. Takagi and S. Uchida, Nature 337 (1989) 345
[7] \qDI, peB 5 (1990) 34
[8] A. Schilling, M. Cantoni, J. D. Guo and H. R. Ott, Nature 363 (1993) 56
[9] J. Akimitsu, S. Suzuki, M. Watanabe and H. Sawa, Jpn. J. Appl. Physics. 27 (1988) L185
[10] A. F. Marshall, R. W. Barton, K. Char, A. Kapitulnik, B. Oh, and R. H. Hammond, Phys. Rev. B 37 (1988) 9353
[11] D. B. Currie, M. T. Weller, P. C. Lanchester and R. Walia, Physica C 224 (1994) 43
[12] D. E. Morris, N. G. Asmar, J. Y. T. Wei, J. H. Nickel, R. L. Sid, J. S. Scott and J. E. Post, Phys. Rev. B 40 (1989) 11406
[13] J. Akimitsu, A, Yamazaki, H. Sawa and H. Fujiki, Jpn. J. Appl. Phys. 26 (1987) L890
[14] S. N. Putilin, E. V. Antipov, O. Chamaissem and M. Marezio, Nature. 362 (1993) 226