Assisted Reproductive Medicine
Assisted Reproductive Medicine
Staff
 | Assoc. Prof. Dr. Junko OOTSUKI E-mail: junkootsuki@(@okayama-u.ac.jp) Specialty: Assisted Reproductive Medicine Basic and applied research on assisted reproductive technologies |
 | Assist. Prof. Dr. Hidetaka TASAKI E-mail: htasaki@(@okayama-u.ac.jp) Specialty: Assisted Reproductive Medicine Development research on advanced technology of mammalian germ cells |
Research Topics
Our assisted reproductive medicine unit was established in 2018. We focus on various methods of assisted reproductive technology including in vitro maturation (IVM), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), the cryopreservation of embryos, oocytes and sperm, and embryo selection. At the same time, we also aim to achieve a greater understanding of certain unknown mechanisms of meiosis, fertilization and embryo development in human.
Research on culturing human embryos and ICSI technology
|
The critical aim of all assisted reproductive technologies is to achieve the fertilization of an oocyte with a sperm. When a patient’s sperm count is too low, and lacks good sperm motility, it becomes necessary to perform ICSI. Although ICSI can improve fertilization rates, pregnancy rates after performing ICSI are known to be lower than pregnancy rates following the performance of IVF. Moreover, we have recently found that meiotic failure at the time of the second polar body extrusion is higher in cases of ICSI than in cases of IVF. It is well known that the rate of chromosomal abnormalities in fertilized oocytes is very high, especially when the women's age is over 42, at which point the chromosomal abnormalities in their fertilized oocytes exceeds 80%. As neither the process of culturing human embryos nor ICSI technology have been perfected at this stage, we aim to develop novel technologies, based on basic experiments employing mouse models. | 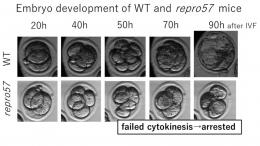 |
The meiotic abnormalities in human oocytes and their mechanisms
|
In human oocytes, we occasionally encounter patients whose oocyte spindles cannot be detected under a polarization microscope, although this is rare. Despite the performance of IVF, ICSI and even oocyte activation, these oocytes do not achieve in normal fertilization. The reasons and mechanisms behind this failure are still unknown. We aim to perform functional analyses of the process of oogenesis, during the first and second meiosis using oocytes from mutant mice, which present with a mutation, leading to genetically developed abnormalities on chromosomal crossing-over during meiosis. In this way, we hope to contribute to the existing body of therapeutic strategies and preventive measures which can be taken against human infertility. * This study is a collaborative work with the unit of Applied Animal Genetics at Okayama University. | 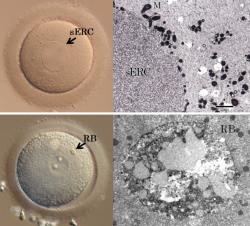 |
The elucidation of the mechanism of dysmorphic phenotypes in human oocytes and preventive measures against those dysmorphisms
|
The dysmorphic phenotypes to be found in human oocytes are unlike any other species, with the exception of chimpanzee oocytes. Among these dysmorphisms, we have been focusing on two phenotypes: smooth endoplasmic reticulum clusters (sERCs) and refractile/lipofuscin bodies. We have found that the incidence of mitotic and meiotic cleavage failure during the second polar body extrusion is significantly higher in oocytes with sERCs than that in oocytes without sERCs. Regarding the refractile body, we have reported that it presents with autofluorescence. Viewed by means of transmitted electron microscopy, the refractile bodies display the conventional morphology of lipofuscin inclusions and consist of a mixture of lipids and dense granule materials. We aim to elucidate the mechanisms of these dysmorphic phenotypes in human oocytes and work towards preventive measures against them. * This study is a collaborative work with the Okayama University Hospital and Fertility clinics. |  |
Publication List
- Otsuki J, Iwasaki T, Katada Y, Tsutsumi Y, Tsuji Y, Furuhashi K, Kokeguchi S, Shiotani M. 2018.A higher incidence of cleavage failure in oocytes containing smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2018;35(5):899-905.
- Otsuki J, Iwasaki T, Tsuji Y, Katada Y, Sato H, Tsutsumi Y, Hatano K, Furuhashi K, Matsumoto Y, Kokeguchi S, Shiotani M. Potential of zygotes to produce live births can be identified by the size of the male and female pronuclei just before their membranes break down. Reprod Med Biol. 2017;12;16(2):200-205.
- Otsuki J, Iwasaki T, Katada Y, Sato H, Furuhashi K, Tsuji Y, Matsumoto Y, Shiotani M. Grade and looseness of the inner cell mass may lead to the development of monochorionic diamniotic twins. Fertil Steril. 2016;106(3):640-4.
- Otsuki J, Nagai Y, Sankai T. Aggregated chromosomes transfer in human oocytes. Reprod Biomed Online. 2014;28(3):401-4.
- Otsuki J, Nagai Y, Matsuyama Y, Terada T, Era S. The redox state of recombinant human serum albumin and its optimal concentration for mouse embryo culture. Syst Biol Reprod Med. 2013;59(1):48-52.
- Otsuki J, Nagai Y, Matsuyama Y, Terada T, Era S. The influence of the redox state of follicular fluid albumin on the viability of aspirated human oocytes. Syst Biol Reprod Med. 2012;58(3):149-53.
- Otsuki J, Nagai Y, Lopata A, Chiba K, Yasmin L, Sankai T. Symmetrical division of mouse oocytes during meiotic maturation can lead to the development of twin embryos that amalgamate to form a chimeric hermaphrodite. Hum Reprod. 2012;27(2):380-7.
- Otsuki J, Nagai Y, Chiba K. Association of spindle midzone particles with polo-like kinase 1 during meiosis in mouse and human oocytes. Reprod Biomed Online. 2009;18(4):522-8.
- Otsuki J, Nagai Y, Chiba K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril. 2009;91(5):1745-9.