Home > About Us > Course of Agrochemical Bioscience > Functional Glycobiochemistry
Functional Glycobiochemistry
Biochemical research on the biofunction of sugar chains: the third class of essential biopolymers
Staffs
 | Professor:Yoshinobu KIMURA, Ph. D. E-mail: yosh8mar@(@cc.okayama-u.ac.jp) (1) Decoding of the structural features and biofunctions of sugar chains or glycans. (2) Application of glycan-biofunction to medical and food science (3) Application of glycan-biofunction to plant breeding through glycogene-manipulation. |
 | Assistant Professor:Megumi MAEDA, Ph. D. E-mail: mmaeda@(@cc.okayama-u.ac.jp) (1) Functional analysis of the immunological activities of bioactive glycans and the application of their functions to medical science. (2) Application of glycan-biofunctions to plant breeding through glycogene-manipulation. |
Research Topics
Glycan (sugar chain or oligosaccharide), as well as nucleic acid (DNA/RNA) and protein, is one of essential biomolecules involved in various biological processes. From yeast or fungus to human, eukaryotic cells produce a large variety of glycans and often covalently couple these glycans to proteins or lipids. The resulting glycoconjugates, such as glycoproteins and glycolipids, play many critical roles in the development or growth of multicellular organisms (animals, plants and insects), and the glycan moieties are relevant to many human health issues and diseases, including viral infection, allergy, and cancer. The research goal of our laboratory is to unravel the latent biofunctions of glycans and apply the decoded glycan-functions to the development of new glycodrugs, functional foods, and plant breeding.
Plant glycobiology
・Functional analysis of oligosaccharides involved in plant differentiation, growth, and fruit maturation. ・Development of new glycotechnology on plant breeding through genetic manipulation of glycogenes involved in glycan metabolism.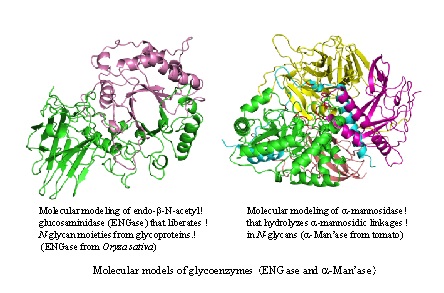
In developing hypocotyls, seeds, and fruits at the maturing stage, free N-glycans (FNG) released from glycoproteins or glycopeptides occur at micro molar concentration. We analyze (1) the biofunctions of FNs involved in plant differentiation, growth, and fruit maturation, (2) chaperon-like functions involved in the protein-folding or -refolding mechanism. Based on the unraveled of the biofunctions of FNGs, we aim to establish a new plant biotechnology to control plant differentiation, growth, and fruit-maturation through artificial regulation of the expression of glycogenes (glycosidases, glycosyltrasferases, and glycan-liberating enzymes).
Glycoimmunolgy
・Analysis of the immunological activities of antigenic glycans of plant or insect origin ・Application of their immunoactivies to the development of glycodrugs.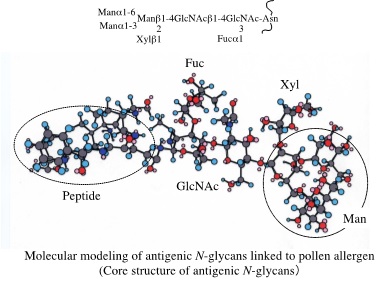
In many case, allergens of plant or insect origin are glycoproteins to which antigenic glycans are linked and these allergens are often referred as glycoallergens. We analyze the chemical structures and immunological activities of these plant glycoallergens. Recently, we have found that plant antigenic glycans suppress the production of Th2 type cytokine, IL-4, from Th2 immune cells. For the application of immunological activity of these plant glycans to the development of glycodrugs, we analyze their cellular immunological activities and synthesize neo-glycopolymers carrying the immunoactive glycans.
Glycotechnology
・Analysis of in vivo / in vitro functions of free N-glycans (FNGs) responsible for correct protein folding and refolding ・Application of the chaperon-like functions of FNGs to the development of glycoreagent stimulating protein correct folding.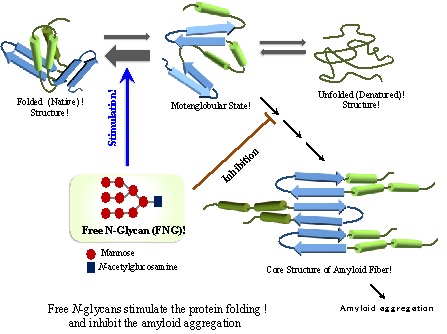
In plant and animal cells or tissues, the liberated asparagine-linked glycans (free N-glycans, FNG) ubiquitously occur. Recently we have found that these FNGs induce the correct protein folding or refolding of denatured or misfolded proteins at millimolar concentration. We analyze the chaperon-like activity of FNGs involved in the protein quality control system working in eukaryotic cells using biochemical and physiochemical strategies. We aim to establish a new concept of glycan function responsible for correct protein folding and apply the chaperon-like function to the development of glycol-reagents stimulating the correct construction of protein 3D structures.
Publications
・ Maeda, M., and Kimura, Y.: Structural and Functional Features of Plant Glycoprotein Glycans. In “Chemistry, Molecular Sciences and Engineering” (Reedijk, J., ed) pp 2-15, Elsevier. (2013) doi: 10.1016/B978-0-12-409547-2.01500-6
・ Maeda, M., Chen, Y., Kumagai-Takei, N., Hayashi, H., Matzuzaki, H., Lee, S., Hirastauka, J-I., Nishimura, Y., Kimura, Y., Ostuki, T.: Alteration of cytoskeletal molecules in a human T cell line caused by continuous exposure to chrysotile asbestos. Immunobiology, 218, 1184-1191 (2013)
・ Maeda, M., Takeda, N., Mano, A., Yamanishi, M., Kimura, M., and Kimura, Y.: Large-scale preparation of Asn-glycopeptide carrying structurally homologous antigenic N-glycan. Biosci. Biotechnol. Biochem. , 77, 1269-1274 (2013)
・ Yokouchi, D., Ono, N., Nakamura, K., Maeda, M., and Kimura, Y.: Purification and characterization of β-xylosidase that is active for plant complex type N-glycans from tomato (Solanum lycopersicum): removal of core α1-3 mannosyl residue is prerequisite for hydrolysis of β1-2 xylosyl residue. Glycoconj. J., 30, 463-472 (2013)


