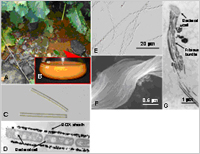
(A) Ocherous deposits ubiquitously seen in water pools.
(B) BIOX deposits collected from a water pool.
(C) BIOX sheaths in the deposit.
(D) A longitudinal section of a BIOX sheath enveloping bacterial cells.
(E) Chain-like BIOX
(F) A twisting BIOX bundle comprised of fibrous materials.
(G) Fibrous bundles connecting to a bacterial cell.
Enlarge Image Fig. 2.
(A) A BIOX sheath covered with a network surface.
(B) Enlarged fibrous matrix of a sheath BIOX with fine surface particles.
(C) Primary particles (ca. 3nm diameter) comprising the matrix.
(D) A computer graphic model showing allocation of FeO6 (yellow) and SiO4 (blue) units in the sheath matrix.
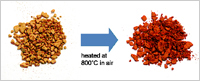
Enlarge Image Fig. 3. Bright yellowish red color of heat-treated BIOX which is expected to yield unprecedented brilliant pigmentation of porcelains.
Enlarge Image Fig. 4. Tubular iron oxide produced by bacteria (diameter is 1/1000 mm)
Enlarge Image
BIOX: Amorphous iron oxide nanostructures of bacterial origin for applications including anodes for Li ion batteries.
Professor Jun Takada, Graduate School of Natural Science and Technology
“Iron-oxidizing bacteria” produce extracellular, uniquely-shaped microsheaths or fibrous bundle nanostructures comprising mainly of iron oxides—known as Biogenous iron oxides (BIOX)—ubiquitously in natural hydrosphere at ambient temperature (Fig. 1).
Although BIOX has been generally recognized as waste, we have studied its properties for as yet unknown potential industrial applications. Our careful and focused studies revealed BIOX matrix to have the following physical properties: (i) an amorphous state; (ii) consist of organic/inorganic hybrid of nanoparticles of approximately 3 nm diameter; (iii) the nanoparticles are composed of many elements, C, O, Fe, Si and P; (iv) inorganic elements are linked via oxygen (Fig. 2).
Importantly, BIOX has a far superior potential (for example a large capacity) as an anode material of Li-ion batteries compared to conventional carbon anodes. In addition, BIOX exhibits an amazing, wide range of functions compared with other materials currently: (i) higher catalytic potential; (ii) higher affinity to human cells; and (iii) brighter color property (Fig. 3). All these characters are superior to those of artificially synthesized iron oxides. We are confident that the eco-friendly, nontoxic, and low-cost BIOX will be a next-generation functional material.
Detailed studies of an isolated strain of one type of the bacteria led us to elucidate the incipient mechanism of BIOX formation. Our experiments showed that extracellular secretion of bacterial polymers triggers deposition and binding of aquatic inorganics such as Fe, Si, and P, which results in the unique organic/inorganic hybrid. Further analysis is in progress for a greater insight into how the mechanism and mode of chemical linkages in the BIOX matrix contribute to the aforementioned functions.
Technical publications
H. Hashimoto et al, “Characteristics of hollow microtubes consisting of amorphous iron oxide nanoparticles produced by iron oxidizing bacteria Leptothrix ochracea,” Journal of Magnetism and Magnetic Materials, 310, 2405, (2007).
T. Sakai et al, “Chemical modification of biogenous iron oxide to create an excellent enzyme scaffold,” Organic Biomolecular Chemistry, 8, 336 (2010).
T. Ema et al, “Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: the dramatic effect of the immobilization support on enzymatic function,” Green Chemistry, 13, 3187 (2011).
T. Suzuki et al, “Environmental microbiology: silicon and phosphorus linkage with iron via oxygen in the amorphous matrix of Gallionella ferruginea stalks”, Applied and Environmental Microbiology, 78, 236 (2012).
M. Furutani et al, “Initial assemblage of bacterial saccharic fibrils and element deposition to form an immature sheath in cultured Leptothrix sp. strain OUMS1”, Minerals, 1, 157, (2011).
K. Mandai et al, “Iron oxide-immobilized palladium catalyst for the solvent-free Suzuki-Miyaura coupling reaction”, Tetrahedron Letters, 53, 329, (2012).
H. Hashimoto et al, “Preparation, microstructure, and colour tone of microtubule material composed of hematite/amorphous-silicate nanocomposite from iron oxide of bacterial origin”, Dyes and Pigments. Available online 6 July 2012. (doi. 10.1016/j.dyepig.2012.06.024).