Enlarge Image
New Route for Synthesis of Roche Intermediate for (–)-Oseltamivir from Replenishable Sources
Teruhiko Ishikawa, Graduate school of Education, Okayama University
The emergence of new flu viruses such as H5N1 had led to increasing concerns about a lethal influenza pandemic. In response to such fears governments can prepare an ample supply or stockpile of oseltamivir phosphate (Tamiflu), an orally active influenza neuraminidase inhibitor currently used for the treatment of influenza A and B viral infection.
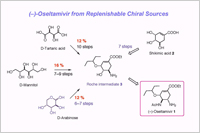
Tamiflu is manufactured by the Roche's method starting from (–)-shikimic acid. However, the starting material is a rare, naturally occurring product, the scarcity of which impedes on-demand production as a generic drug. Hence, developing alternative effective methods to synthesize Tamiflu with more reliable sources of starting materials are highly desired. Here, we disclose a new, practical route to the Roche intermediate for Tamiflu synthesis.
Our synthesis method has the following features:
(1) The method utilizes inexpensive and replenishable natural sources such as D-mannitol, D-tartaric acid, or D-arabinose as starting materials, and is thus free from the inevitable disadvantages that the shikimic acid route presents.
(2) The method enables the synthesis Roche intermediate, and subsequently, the synthesis of Tamiflu using established plant scale processes.
(3) Synthetic transformations are conducted in the range of 0 ℃ to room temperature using safe and inexpensive reagents, which underscores the practicality of this method for application to process chemistry.
US Patent US8524940 B2 (Sep. 3, 2013)
Contact: Mototaka Senda, Ph.D.,
US & EU Representative of Intellectual Property Office,
Okayama University, 2450 Peralta Blvd. #119, Fremont, CA 94536, USA
Email: takasenda@okayama-u.ac.jp