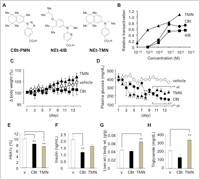
Dr. Kakuta and his colleagues have synthesized and selected two RXR partial-agonists CBt-PMN (EC50 = 143 nM, Emax = 75%)(ref. 2-5) and 6-[ethyl (4-isobutoxy-3-isopropylphenyl)amino]-3-pyridinecarboxylic acid (NEt-4IB, EC50 =169 nM , Emax = 55%)(ref. 6-8) and demonstrated that these compounds showed lower blood glucose levels on animal studies without adverse effects such as weight gain, elevated TG levels in blood or hepatomegaly (Figure 2). This study of the RXR partial-agonists supports our hypothesis on "Westernized Kampo Medicine", which could make a difference in new drug discovery research.
Enlarge Image Figure 2. A) Chemical structures of CBt-PMN (CBt), NEt-4IB (4IB) and RXR full-agonist NEt-TMN (TMN). B) Dose-dependence of RXRα agonist activities of each compound. The transactivation activity is shown as relative activity based on the luciferase activity of 1 μM RXR full-agonist bexarotene taken as 1.0. C) − H) Evaluation of antitype 2 diabetes effects of repeated oral administration of CBt and TMN at 10 mg/kg/day to male KK-Ay mice for 14 consecutive days. Data for vehicle control, CBt and TMN were taken from ref 2. Data shown are the average (n = 4−7) ± SEM and analyzed by one-way ANOVA followed by Bonferroni test. Significant differences: * p < 0.05 vs vehicle. ** p < 0.01 vs vehicle. These data were quoted from reference 2 and modified.
Enlarge Image
Synthesis of novel homeostasis modulators by "Westernized Kampo Medicine"
—Retinoid X Receptor Partial-Agonists Exert Anti-type 2 Diabetes Effects with Less Adverse Effects than Full-Agonists—
"Westernized Kampo Medicine" is a novel approach in modern medicine, defined by Dr. Hiroki Kakuta, that intends to exhibit the effects of Japanese Kampo Medicine with small molecules (Ref. 1). Japanese Kampo Medicine was developed in Japan, branching from traditional Chinese Medicine (Oriental Medicine). In contrast to Western Medicine, which has a well-regarded therapeutic method of treating diseases by using drugs focused on target molecules such as receptors or enzymes specifically related to each disease, Oriental Medicine is a systematic treatment based on consideration of a patient's homeostatic condition and environmental factors to determine a patient's well-being. In particular, Chinese herbs are one of the tools used for treatments in Oriental Medicine. Recent common diseases such as diabetes, Alzheimer's Disease, and cancer are considered to be closely related to patients' life styles, and are expected to be diagnosed and be treated by "Westernized Kampo Medicine".
Dr. Kakuta has recently demonstrated this new concept from his exploratory research on the study of Retinoid X Receptor (RXR). By this concept, a new drug, a small molecule as opposed to a Chinese herb of Oriental Medicine, was synthesized, selected and proved to work as a partial-agonist to its target molecule, which exerts significant efficacy with less adverse effects.
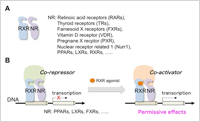
RXRs act as dimers that consist of peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs) or other receptors (Figure 1). PPARs and LXRs are known to control glucose/lipid metabolism and develop anti-inflammatory effects through the suppression of NF-κB. The hetero-dimers can be activated by RXR agonists alone (Permissive Effect). It is reported that RXR agonists have anti-diabetic and anti-inflammatory effects. However, the RXR agonists reported previously are full-agonists and lead to the elevation of the triglyceride (TG) level in blood, hypothyroidism, weight gain, and hepatomegaly. Dr. Kakuta and his colleagues hypothesized that these adverse effects are caused by the excessive activation of RXR as a full-agonist, and that more moderate activation of RXR as a partial-agonist is sufficient for exhibiting the desired drug efficacy.
Our synthesis method has the following features:
1. Expert Opin. Ther. Pat. 24, 443–52, (2014).
2. ACS Med. Chem. Lett., 3, 427–43 (2012).
3. J. Med. Chem., 56, 1865–77, (2013).
4. JP5255994 B2
5. JP2013-177329A
6. Manuscript in preparation
7. JP4691619 B2, US8389538 B2, EP2116523 A1
8. JP2014-076953A
Contact: Mototaka Senda, Ph.D.,
US & EU Representative of Intellectual Property Office,
Okayama University, 2450 Peralta Blvd. #119, Fremont, CA 94536, USA
Email: takasenda@okayama-u.ac.jp