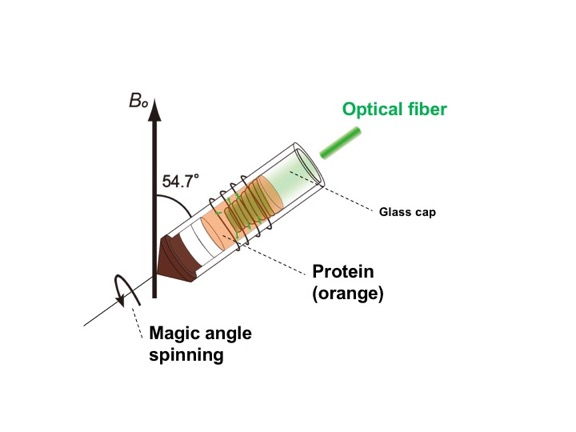
Enlarge Image Fig.2. In situ photo-irradiation solid-state NMR apparatus with magic angle spinning arrangement.
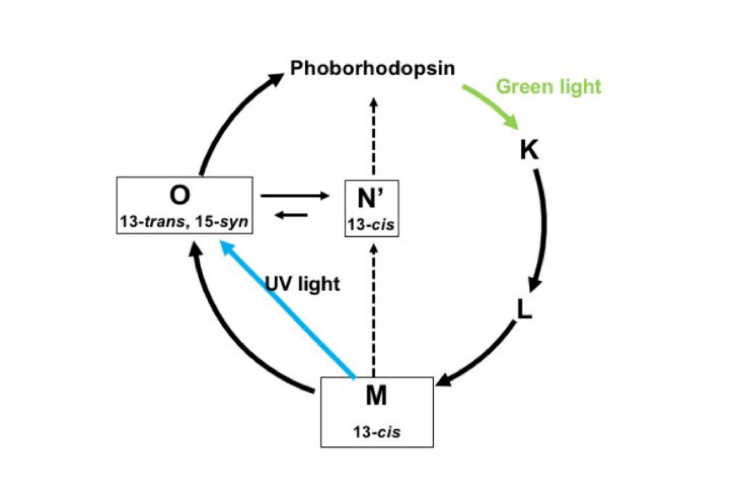
Enlarge Image Fig. 3. Photoreaction cycle of retinal of phoborhosdopsin. NMR signals of short-lived M-, O- and N’ intermediates were observed in the first time in this research highlight.
Enlarge Image
In-situ photo-irradiation solid-state NMR for detecting the functional changes of photoreceptor-protein structure
Microbial rhodopsin is an attractive target protein in biophysical chemistry in order to understand the relationships between structure, dynamics, and functions.
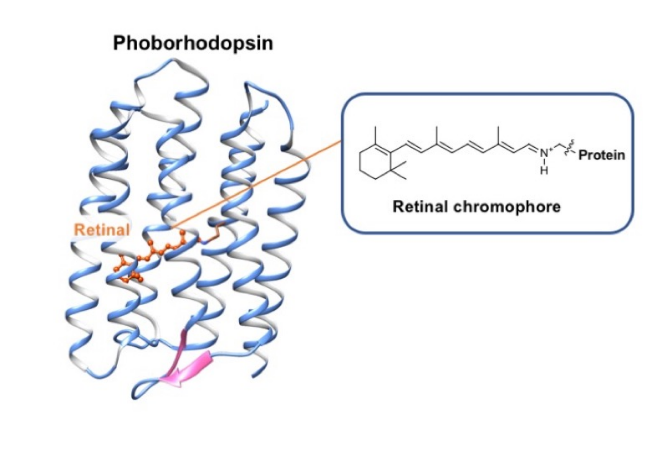
Although a microbial rhodopsin “phoborhodopsin” is a negative phototaxis receptor protein with a retinal chromophore whose function has been well studied by Yuki Sudo, the structure of the chromophore, especially in the short-lived photo-intermediates, are not understood (Fig. 1).
Now, Akira Naito, Izuru Kawamura and colleagues at Yokohama National University, Kobe Pharmaceutical University, Hokkaido University, and Okayama University have shown that in-situ photo-irradiation solid-state NMR can detect several early photo-intermediates with structure of chromophore in phoborhodopsin.
Phoborhodopsin with 13C stable isotope-labeled retinal was reconstituted into lipid bilayers. The sample was packed into an NMR tube, and the sample was photo-irradiated under the magic angle spinning condition, which is a high-resolution technique. The in-situ photo-irradiation solid-state NMR apparatus allows irradiation of the samples with extremely high efficiency and enables observation of short-lived photo-intermediates in the stationary trapped state (Fig. 2).
The chemical shifts of the 13C NMR signal of 13C labeled retinals were carefully detected and analyzed under photo-irradiation. Consequently, short-lived M- and O-intermediates were detected with a newly detected N’-intermediate, which turned out to have been transformed from the M-intermediate (Fig. 3).
The structures of retinals for the short-lived M-, O- and N’-intermediates were revealed to be (13-cis, 15-syn), (13-trans, 15-syn) and (13-cis), respectively. This challenging photo-irradiation NMR spectroscopy provides an opportunity to detect the dynamic conformational change of proteins enclosing retinals in relation with function. Eventually, it will lead to understanding the photoactivation mechanism at the molecular level.
Highlighted paper
- Y. Makino, I. Kawamura, T. Okitsu, A. Wada, N. Kamo, Y. Sudo, K. Ueda, A. Naito. (2018) Retinal configuration of ppR intermediates revealed by photoirradiation solid-state NMR and DFT. Biophys. J. 115, 72-83.
Related publications
- Y. Sudo and J. L. Spudich (2006) Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor. Proc. Natl. Acad. Sci., 103, 16129–16134.
- Y. Sudo, Y. Furutani, H. Kandori, and J.L. Spudich. (2006) Functional Importance of the Interhelical Hydrogen Bond between Thr204 and Tyr174 of Sensory Rhodopsin II and Its Alteration during the Signaling Process. J. Biol. Chem. 281, 34239-34245.
- H. Yomoda, Y. Makino, Y. Tomonaga, T. Hidaka, I. Kawamura, T. Okitsu, A. Wada, Y. Sudo, A. Naito. Color Discriminating Retinal Configurations of Sensory Rhodopsin I by Photo-Irradiation Solid State NMR Spectroscopy. (2014) Angew. Chem. Int. Ed. 53 (27) 6960-6964.
- Y. Tomonaga, T. Hidaka, I. Kawamura, T. Nishio, K. Osawa, T. Okitsu, A. Wada, Y. Sudo, N. Kamo, A. Ramamoorthy, A. Naito.
- An Active Photo-Receptor Intermediate Revealed by in-situ Photo-Irradiated Solid-State NMR Spectroscopy. (2011) Biophys. J. 101 (10) L50-52.
- Affiliations: Research Institute for Interdisciplinary Science, Okayama University.
- Okayama University Scientific Achievement Repository: http://ousar.lib.okayama-u.ac.jp/56413