Synthesis of novel anti malarial agents
May 29, 2013
Researchers from Okayama University Graduate School of Natural Science and Technology have synthesized new structural compounds of antimalarial agents and elucidated their mode of action.
The new compounds could lead to develop a new anitmalarial drug that replaces Chloroquine, the drug commonly used to treat malaria.
The findings were published in the journal European Journal of Medicinal Chemistry on April 15, 2013.
http://www.sciencedirect.com/science/article/pii/S0223523413002328
Prof. Inokuchi and his colleagues in the collaborative research group have synthesized new compounds that have higher antimalarial activities than those of current drugs and studied their mode of action.
Malaria is one of three major infectious diseases in the world, and a main cause of death in the tropical areas.
Currently, drugs for malaria in the clinical field are: (1) quinine group compounds, (2) combinations of folic acid antagonists, and (3) artemisinin-related compounds. The research group focused on the extract from the root of Cryptolepis sanguinolenta that is a native plant used for malaria and cancer treatment in Western and Central Africa. The group used indoloquinolines, the plant component, as a lead compound, and synthesized the derivatives for antimalarial agents. The group has established a simple and large-scalable synthesis of the indoloquinoline skeleton. The modifications were carried out by introducing ester groups at the C2 position on the skeleton and amino groups at the C11 position (Figure1, Compounds I and II). The antiplasmodial activities of the modified derivatives and the cytotoxic activity were evaluated. The test results showed that in vitro, Compound IIa had higher antiplasmodial activity (IC50) against the NF54 strain than chloroquine and a low cytotoxic activity.
Furthermore, the compounds were tested forβ-haematin inhibition and they were found to be more active than chloroquine and artemisinin. Structure activity relationship studies exposed an interesting linear correlation between polar surface area of the molecule andβ-haematin inhibition for this series.
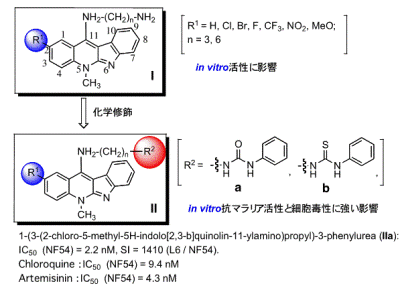
Figure1.Structures of indoloquinolines for antimalarial agents and their antiplasmodial activites
expected to lead to the development of a new antimalarial agent to be used against Plasmodium falciparum, which is sometimes resistant to chloroquinoline and/or artemisinin.
Contact Information:
Mototaka Senda, Ph.D.
Intellectual Property Office, Organization for Research Promotion and Collaboration
US Representative, Fremont, California USA
TEL: 1-510-797-0907
Email: takasenda@okayama-u.ac.jp
Tsutomu Inokuchi, Ph.D.
Graduate School of Natural Science and Technology, Okayama University, Okayama Japan