Four-Electron Oxidative Dehydrogenation Induced by Proton-Coupled Electron Transfer in Ruthenium (III) Complex
September 05, 2013
Researchers in the Okayama University Graduate School of Natural Science and Technology have revealed that four-electron oxidative dehydrogenation is induced by proton-coupled electron transfer (PCET) in a given ruthenium (III) complex with the addition of a base under mild conditions.
The findings were published online in the journal Inorganic Chemistry on August 22, 2013.
http://pubs.acs.org/doi/abs/10.1021/ic401667v
R. Mitsuhashi, Ph.D. candidate and his research group have examined the structures and properties of ruthenium (II or III) complexes with tetrahydropyrimidyl groups. One result is that once ruthenium (II) complex is oxidized and turned into the ruthenium (III) complex, the ruthenium (III) complex easily releases electrons and protons with the
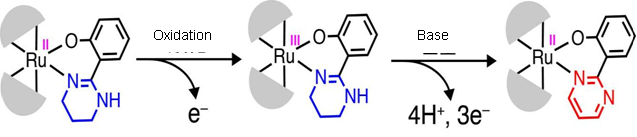
Figure 1
Generally, the oxidation from tetrahydropyrimidyl group to pyrimidyl group needs a strong oxidizing agent and high temperature. This study showed that a multistage oxidative reaction could be done under mild conditions by PCET in the metal complexes.
This PCET reaction is expected to apply to the water oxidation that is a key reaction in the artificial photosynthesis process.
This research was funded by the Japan Society for the Promotion of Science (JSPS) [#25410670, #258041]
Contact Information:
Mototaka Senda, Ph.D.
US Representative
Intellectual Property Office, Organization for Research Promotion and Collaboration, Okayama University
Fremont, California USA
TEL: 1-510-797-0907
Email: takasenda@okayama-u.ac.jp
Takayoshi Suzuki, Ph.D.
Graduate School of Natural Science and Technology, Okayama University, Okayama Japan