 |
||||||||||
Oxycarbonate cuprate called Sr2CuO2(CO3) was reported in 1988. After the report, it was found that the crystal structure has CuO2 plane which consists of Cu and O like copper oxide superconductor, and stacks Sr2CO3 and CuO2 plane alternately. Since the discovery of oxycarbonate superconductor, (Ba0.55Sr0.45)2Cu1.1O2.2+Â(CO3)0.9 with 30 K of superconductive transition temperature, our laboratory has developed oxycarbonate superconductors. Here, let us introduce the oxycarbonate superconductors discovered by this laboratory.
1. 1992 (Y,Ca)Sr2Cu3-x(CO3)xOy Tc=63K
2. 1992 (Bi,Pb)-Sr-Cu-(CO3)-O Tc = 41K , 54K
3. 1993 Ca(Ca,Sr)2Cu2(CO3)1-x(BO3)x Tc=33K , 55K , 105K , 115K
4. 1993 Tl(Ba,Sr)4Cu2(CO3)O7-Â Tc=73K
5. 1993 Hg(Ba,Sr)4Cu2(CO3)O6-Â Tc=67K
 Development of the
oxycarbonate superconductors began with the concept of the block layer by
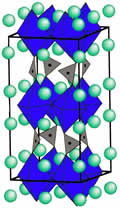
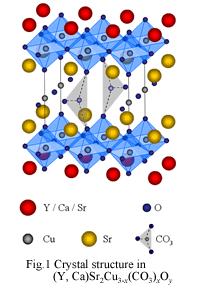
Tokura and Arima at the University of Tokyo. Our laboratory regards Sr2CO3 as a new block layer after the discovery of Sr2CuO2(CO3), and composed oxycarbonate superconductors under partial pressure of a carbonic acid. Fig.1 is the
crystal structure of (Y,Ca)Sr2Cu3-x(CO3)xOy. Replacing Y3+ by
Ca2+, this substance shows the superconductor with Tc = 63 K. Moreover, as it replaces Y by lanthanoids,
such as Er, Ho, Dy, Gd, Eu, and Sm, it becomes a
superconductor with Tc ~ 50 K. As for the crystal structure,
some Cu-O chains are replaced with the carbonate ion, because the size of carbonate ion and the size of the
copper-oxide perovskite are almost the same. Therefore we expect many cuprates show superconductors composed under partial
pressure of a carbonic acid.
Development of the
oxycarbonate superconductors began with the concept of the block layer by
Tokura and Arima at the University of Tokyo. Our laboratory regards Sr2CO3 as a new block layer after the discovery of Sr2CuO2(CO3), and composed oxycarbonate superconductors under partial pressure of a carbonic acid. Fig.1 is the
crystal structure of (Y,Ca)Sr2Cu3-x(CO3)xOy. Replacing Y3+ by
Ca2+, this substance shows the superconductor with Tc = 63 K. Moreover, as it replaces Y by lanthanoids,
such as Er, Ho, Dy, Gd, Eu, and Sm, it becomes a
superconductor with Tc ~ 50 K. As for the crystal structure,
some Cu-O chains are replaced with the carbonate ion, because the size of carbonate ion and the size of the
copper-oxide perovskite are almost the same. Therefore we expect many cuprates show superconductors composed under partial
pressure of a carbonic acid.
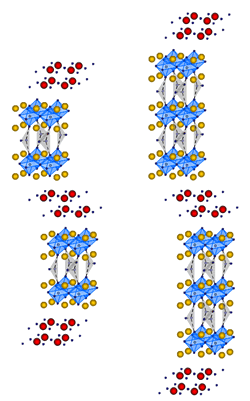 The feature of oxycarbonate superconductors is
combination of the block layers which supply apex oxygen in spite of the fact that it was considered cuprates could not keep such block layers. Therefore in case of oxycarbonate superconductors, any combination of
block layers is permitted. It is expexted that many approaches for depeloping new superconductors. Fig.2-1 and 2-2
are the crystal structures of (Bi,Pb)2Sr2+nCu1+n(CO3)nO6+2n. Fig.2-1 corresponds to the same crystal structure of Bi-2223 and it is the
case of n=1. Moreover, the case of n=2
is shown in Fig.2-2, it is the same structure with Bi-2245. CuO2-plane without apex oxygen is replaced by the carbonate
ion. These have unstable structure stacking CuO6. In the chemical viewpoint, it is not known whether the apex oxygen shared with this
carbonate ion is regarded as normal apex
oxygen. Therefore, this is very interesting that such structures are permitted. This system
shows superconductivity in replacing Bi3+ by Pb2+. This
superconductive transition temperature is about 41[K] in the case of n=1
((Bi,Pb)2Sr3Cu2(CO3)O8) and is about 54[K] in the case of n=2 ((Bi,Pb)2Sr4Cu3(CO3)2O10). Although it is a low value compared with the
usual transition temperature such as Bi-2223, this never means that the
carbonates never show high temperature superconductivity. The answer is shown in the next chapter about replacement by borate.
The feature of oxycarbonate superconductors is
combination of the block layers which supply apex oxygen in spite of the fact that it was considered cuprates could not keep such block layers. Therefore in case of oxycarbonate superconductors, any combination of
block layers is permitted. It is expexted that many approaches for depeloping new superconductors. Fig.2-1 and 2-2
are the crystal structures of (Bi,Pb)2Sr2+nCu1+n(CO3)nO6+2n. Fig.2-1 corresponds to the same crystal structure of Bi-2223 and it is the
case of n=1. Moreover, the case of n=2
is shown in Fig.2-2, it is the same structure with Bi-2245. CuO2-plane without apex oxygen is replaced by the carbonate
ion. These have unstable structure stacking CuO6. In the chemical viewpoint, it is not known whether the apex oxygen shared with this
carbonate ion is regarded as normal apex
oxygen. Therefore, this is very interesting that such structures are permitted. This system
shows superconductivity in replacing Bi3+ by Pb2+. This
superconductive transition temperature is about 41[K] in the case of n=1
((Bi,Pb)2Sr3Cu2(CO3)O8) and is about 54[K] in the case of n=2 ((Bi,Pb)2Sr4Cu3(CO3)2O10). Although it is a low value compared with the
usual transition temperature such as Bi-2223, this never means that the
carbonates never show high temperature superconductivity. The answer is shown in the next chapter about replacement by borate.
3. Ca(Ca,Sr)2Cu2(CO3)1-x(BO3)x
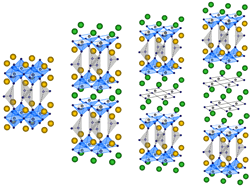 In case of exygen deficit type block layer structure in Ca system, the multilayer structure is permitted. It is possible to increase the number of CuO2 planes in a unitcell. Our laboratory succeeded in compounding a substance Ca(Ca,Sr)2+nCu1+n(CO3)1-x(BO3)xOy (n=1,2,3) with stacking structure as shown in Fig.3. The carrier control is performed by replacing CO3 by BO3. The
chemical bond and the size of carbonate ion are
similar, but the balence between the two is different. Therefore this is a very effective method. The superconductive transition
temperature is 55K (n=1), and 105K (n=2)
and 115K (n=3), respectively. In the case of n=1, if we compose not using Ca, but using Ba, the superconductive
transition temperature goes up to 60K. This is
because the CuO2-plane is smoothed by replacing by a large ion radius element, Ba. In the case of n=3,
it realizes high transition temperature, and it is one of the highest Tc superconductors,
excepting for those include dangerous elements such as Hg or Tl. It is thought that this system is suitable for
carrier control because carbonate ion can be replaced by borate
ion. It is expected that the copper oxide superconductor containing
carbonate ion with multilayer structure will exceed the
transition temperature of Hg system.
In case of exygen deficit type block layer structure in Ca system, the multilayer structure is permitted. It is possible to increase the number of CuO2 planes in a unitcell. Our laboratory succeeded in compounding a substance Ca(Ca,Sr)2+nCu1+n(CO3)1-x(BO3)xOy (n=1,2,3) with stacking structure as shown in Fig.3. The carrier control is performed by replacing CO3 by BO3. The
chemical bond and the size of carbonate ion are
similar, but the balence between the two is different. Therefore this is a very effective method. The superconductive transition
temperature is 55K (n=1), and 105K (n=2)
and 115K (n=3), respectively. In the case of n=1, if we compose not using Ca, but using Ba, the superconductive
transition temperature goes up to 60K. This is
because the CuO2-plane is smoothed by replacing by a large ion radius element, Ba. In the case of n=3,
it realizes high transition temperature, and it is one of the highest Tc superconductors,
excepting for those include dangerous elements such as Hg or Tl. It is thought that this system is suitable for
carrier control because carbonate ion can be replaced by borate
ion. It is expected that the copper oxide superconductor containing
carbonate ion with multilayer structure will exceed the
transition temperature of Hg system.
 Although Tl is
very a strong toxic element, it is known that the copper oxide containing Tl
shows very high transition temperature. Therefore the material design in Tl system has been
performed earnestly. As for superconductors containing
carbonate ion, Tl0.5Pb0.5Sr4Cu2(CO3)Oy was discovered. The superconductive transition temperature is 70 [K], and
this discovery enhanced the material design containing the carbonate ion in Tl
system. Our laboratory discovered a superconductor with very unique super-lattice structure, Tl(Ba,Sr)4Cu2(CO3)O7-Â. This substance tends to be overcareer because
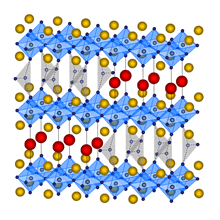
of much oxygen deficit. Therefore without a carrier doping, it has 73[K] of superconductive transition temperature. Fig.4 is the crystal structure. It
is found that the substance has the periodic structure called
(C-C-C)-(Tl-Tl-Tl)-(C-C-C) in the direction of a-axis. This periodic structure is realized by the amount of substitution of Ba. Moreover, as Sr and Ba is a divalent, Ba substitution does not result in carrier
control. However, transition temperature changes with the
amount of Ba substitution. This is because the amount of oxygen changed with substitution. This is very interesting because the periodic structure is caused by Ba substitution and crystal structure is changed by the amount of oxygen.
Although Tl is
very a strong toxic element, it is known that the copper oxide containing Tl
shows very high transition temperature. Therefore the material design in Tl system has been
performed earnestly. As for superconductors containing
carbonate ion, Tl0.5Pb0.5Sr4Cu2(CO3)Oy was discovered. The superconductive transition temperature is 70 [K], and
this discovery enhanced the material design containing the carbonate ion in Tl
system. Our laboratory discovered a superconductor with very unique super-lattice structure, Tl(Ba,Sr)4Cu2(CO3)O7-Â. This substance tends to be overcareer because
of much oxygen deficit. Therefore without a carrier doping, it has 73[K] of superconductive transition temperature. Fig.4 is the crystal structure. It
is found that the substance has the periodic structure called
(C-C-C)-(Tl-Tl-Tl)-(C-C-C) in the direction of a-axis. This periodic structure is realized by the amount of substitution of Ba. Moreover, as Sr and Ba is a divalent, Ba substitution does not result in carrier
control. However, transition temperature changes with the
amount of Ba substitution. This is because the amount of oxygen changed with substitution. This is very interesting because the periodic structure is caused by Ba substitution and crystal structure is changed by the amount of oxygen.
 The new
superconductor containing Hg, HgBa2CuO4+Â, is discovered in 1993. This substance shows the superconductivity at 94 [K]. It has only one CuO2-plane
in unit cell. HgBa2Ca2Cu3Oy, which can increase the number of CuO2-plane
shows superconductivity at 134[K] and has the present highest transition temperature. This substance increases the transition temperature up to 164[K] by
applying pressure. This is because the new type block layer HgBaO2+Âwhich lacks most of the
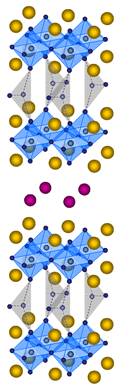
oxygen in HgO. With the new block layer this HgBaO2+Â and the block layer containing carbonate ion called Sr2CO3,
our laboratory discovered HgSr2Ba2Cu2(CO3)O6+Â shown in Fig.5.
CuO2-plane is not a perfect 2-dimensional plane, but very rippled structure. By the sample which administered oxygen annealing,
superconductive transition temperature is about 67[K], and it has become
a comparatively low value in Hg system. However, it is also possible that it has the high transition
temperature 67[K] although CuO2-plane is rippled. We can
say that the substance containing carbonate ion in Hg system has high transition temperature, judging from the result of
the usual Hg system copper oxide superconductor.
The new
superconductor containing Hg, HgBa2CuO4+Â, is discovered in 1993. This substance shows the superconductivity at 94 [K]. It has only one CuO2-plane
in unit cell. HgBa2Ca2Cu3Oy, which can increase the number of CuO2-plane
shows superconductivity at 134[K] and has the present highest transition temperature. This substance increases the transition temperature up to 164[K] by
applying pressure. This is because the new type block layer HgBaO2+Âwhich lacks most of the
oxygen in HgO. With the new block layer this HgBaO2+Â and the block layer containing carbonate ion called Sr2CO3,
our laboratory discovered HgSr2Ba2Cu2(CO3)O6+Â shown in Fig.5.
CuO2-plane is not a perfect 2-dimensional plane, but very rippled structure. By the sample which administered oxygen annealing,
superconductive transition temperature is about 67[K], and it has become
a comparatively low value in Hg system. However, it is also possible that it has the high transition
temperature 67[K] although CuO2-plane is rippled. We can
say that the substance containing carbonate ion in Hg system has high transition temperature, judging from the result of
the usual Hg system copper oxide superconductor.