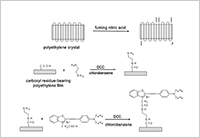
Enlarge Image Figure 2. Manufacturing process of photoelectric dye-coupled polyethylene film (OURePTM): a novel type of retinal prosthesis.
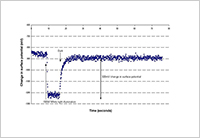
Enlarge Image Figure 3. Fast on-off response to light of electric potentials on the surface of photoelectric dye-coupled polyethylene film (OURePTM): a novel type of retinal prosthesis.
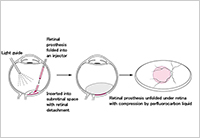
Enlarge Image Figure 4. Schematic drawing of vitreous surgery to implant a sheet of photoelectric dye-coupled polyethylene film (OURePTM) , a novel type of retinal prosthesis, beneath the retina.
Enlarge Image
Photoelectric dye-coupled thin film as a novel type of retinal prosthesis
Eye doctor Dr. Toshihiko Matsuo and polymer science engineer Dr. Tetsuya Uchida have been developing a new type of retinal prosthesis that is based on a photoelectric dye. The photoelectric dye is an organic molecule that absorbs light and converts light energy into electric potentials. The dye molecules are coupled to the surface of a film made of polyethylene. The polyethylene film (or polymer) is a biologically safe and stable material which is used, for example, as a component of artificial joints. The photoelectric dye-coupled polyethylene film, called Okayama University-type retinal prosthesis or OURePTM, can be implanted beneath the retina as a substitute for photoreceptor cells.
The photoreceptor cells in the retina absorb light and generate membrane potential changes as an initial process in sight. Patients with a hereditary disease called retinitis pigmentosa, gradually lose the photoreceptor cells in their lifetime and become totally blind. These vision-impaired patients are known to maintain other retinal neurons that connect to the brain. Thus, the implantation of something artificial called retinal prosthesis to replace the lost photoreceptor cells, would lead to the recovery of vision in blind patients.
A prevailing type of retinal prosthesis is the so-called “camera-image-capture and electrode-array output system”. The image is captured by a digital video camera attached to glasses, and converted to electric signals. These signals are transmitted to a receiver implanted in the body, and finally, electric currents are outputted from an electrode array that is implanted around the degenerated retina. In 2013, Argus IITM Retinal Prosthesis System, by Second Sight, Inc., which uses this camera-capture and electrode-array system, was approved by the US Food and Drug Administration (FDA).
Okayama University-type retinal prosthesis—OURePTM—would provide the following advantages over the Argus IITM Retinal Prosthesis System. First of all, OURePTM does not require a camera or data processing system, or wiring to the retina. A single sheet of OURePTM would be implanted into the subretinal space by currently-used standard vitreous surgery, just as to treat retinal detachment. A large size of the thin film, up to 10 mm in diameter, could be implanted in the eye, which would provide a wide visual field. Dye molecules in high density on the polyethylene surface work as both an image (light)-receiver and a neuron-stimulator, leading to high resolution of images. In contrast, the Argus IITM System with 60 electrodes provides low resolution of images.
The biological safety of OURePTM has been already proven by standardized tests, based on ISO 10993, “Biological evaluation of medical devices”. In addition, the photoelectric dye, used for OURePTM, has no toxicity at all. Rats with retinitis pigmentosa, called RCS rats, had their vision restored by subretinal implantation of OURePTM. Manufacturing and quality control has been established at the laboratory of Polymer Materials Science in a Faculty-of-Engineering Building. Dr. Matsuo and Dr. Uchida are now preparing a first-in-human clinical trial at Okayama University Hospital, in consultation with Pharmaceuticals and Medical Devices Agency (PMDA, counterpart of US FDA), based on the Pharmaceutical Affairs (Pharmaceuticals and Medical Devices) Act in Japan.
1.Matsuo T, Dan-oh Y, Suga S (Inventors). Agent for inducing receptor potential. Assignee: Okayama University. United States Patent. Patent No.: US 7,101,533 B2. Date of Patent: Sep. 5, 2006.
2.Matsuo T, Uchida T, Takarabe K. Safety, efficacy, and quality control of a photoelectric dye-based retinal prosthesis (Okayama University-type retinal prosthesis) as a medical device. J Artif Organs 2009;12:213-225.
3.Alamusi, Matsuo T, Hosoya O, Tsutsui MK, Uchida T. Behavior tests and immunohistochemical retinal response analyses in RCS rats with subretinal implantation of Okayama University-type retinal prosthesis. J Artif Organs 2013;16 :343-351.
★Contact: Mototaka Senda, Ph.D.,
US & EU Representative of Intellectual Property Office,
Okayama University, 2450 Peralta Blvd. #119, Fremont,
CA 94536, USA
Email: takasenda@okayama-u.ac.jp