Enlarge Image
An interesting twist on supercooled liquid water
Water is known to have various anomalous properties, and they are especially prominent below room temperature. For example, liquid water exhibits expansion when it is cooled below 4°C, and it keeps expanding when it is supercooled below the freezing point, 0°C. Finding a unified explanation of the anomaly of water is a long-standing challenge. It is important not only in physical chemistry but also in various other fields of science, such as biology and astronomy.
Using computer simulations Masakazu Matsumoto and his collaborators, Takuma Yagasaki and Hideki Tanaka, succeeded in detecting the molecular ordering in supercooled liquid water. Liquid water at low temperatures is not a homogeneous liquid but a dynamic aggregate of ordered nano grains. “Liquid water seems smooth and simple,” says Matsumoto, an associate professor at Okayama University. “But it conceals complexity behind its appearance.”
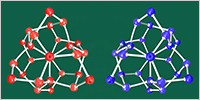
The team obtained many configurations of water molecules in liquid water by a computational technique called molecular dynamics, and analyzed the local structures in water using a pattern-matching technique. Finally, they found that the special structure named “extended polytope” is the most abundant at low temperatures. This tiny and twisted structure is as stable as crystal ice and exists as nano-sized grains. The grains are chiral, i.e. there are two types of the structure that are mirror images of each other.
“Emergence of the chiral order is quite surprising,” says Yagasaki, an assistant professor at Okayama University. “It has been thought that the liquid structure of water becomes ice-like when it is supercooled, but no known crystal ice structure possesses chirality at any length scale.”
Their new analysis method enables a new way of recognizing order in the water molecules around biomolecules and in amorphous ice. The twisted structures of water might induce a new kind of interaction between the molecules dissolved in water, and might hinder ice nucleation.
“Water plays a central role in living things.” says Dr. Tanaka, a professor at Okayama University. “Our discovery will allow us to reconsider the synergy between water and life from a different angle.”
Reference:
・ Authors: Masakazu Matsumoto, Takuma Yagasaki, and Hideki Tanaka.
・ Title of original paper: Chiral ordering in supercooled liquid water and amorphous ice.
・ Journal, volume, pages and year: Physical Review Letters 115, 197801 (2015).
・ Digital Object Identifier (DOI): 10.1103/PhysRevLett.115.197801
・ http://ousar.lib.okayama-u.ac.jp/metadata/53939
・ Journal website: http://link.aps.org/doi/10.1103/PhysRevLett.115.197801
・ Affiliations: Department of Chemistry, Faculty of Science, Okayama University.
・ Department website: http://www.chem.okayama-u.ac.jp
Reference(e-Bulletin) :
OKAYAMA UNIV. e-Bulletin. Adsorption mechanism of inhibitor and guest molecules on the surface of methane hydrate. Vol.13, Dec, 2015.
http://www.okayama-u.ac.jp/user/kouhou/ebulletin/research_highlights/vol13/highlights_004.html
OKAYAMA UNIV. e-Bulletin. Theoretical physics: Demystifying the molecular mechanisms of the initial stages of how ice melts. Vol.4, Sep, 2013.
http://www.okayama-u.ac.jp/user/kouhou/ebulletin/research_highlights/vol4/highlights_004.html