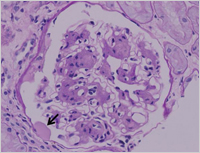
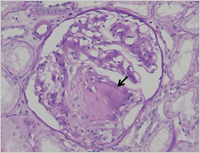
Enlarge Image Figure 2 Characteristic nodular lesion is demonstrated in the glomerulus (arrow). (PAS stain)
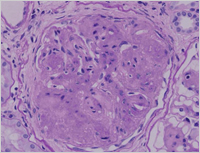
Enlarge Image Figure 3 The glomerulus demonstrats global sclerosis, i.e. glomerulosclerosis, and the glomerular capillary structures are not seen. (PAS stain)
Enlarge Image
Inflammation and diabetic nephropathy
One of the most challenging issues in clinical nephrology is the relentless and progressive increase in the number of patients with end-stage renal disease (ESRD) worldwide. Among the various kinds of kidney diseases, the impact of diabetic nephropathy on the increasing population of patients with chronic kidney disease (CKD) and ESRD is enormous. Diabetic nephropathy is characterized by the accumulation of extracellular matrix in glomeruli—called exudative— diffuse and nodular lesions. These pathlogical changes are finally followed by glomerulosclerosis and interstitial fibrosis, and in such situations, the patients inevitably undergo dialysis therapies and renal transplantation to survive.
The three major classical pathways in the progression of diabetic nephropathy are (1) the activation of polyol and protein kinase C (PKC) pathways;( 2) the formation of advanced glycation end-products; and( 3) intraglomerular hypertension induced by glomerular hyperfiltraion. In the upstream of the three pathways, hyperglycemia is the major driving force for progression to end-stage renal diseases from diabetic nephropathy. Downstream of the three major pathways, many researchers are convinced that the inflammation pathways play central roles in the progression of diabetic nephropathy and the identification of inflammatory molecules may lead to the development of therapeutic strategies.
Some of the molecules related to the inflammation pathways in diabetic nephropathy include transcription factors, proinflammatory cytokines, chemokines, adhesion molecules, Toll-like receptors, adipokines, and nuclear receptors, which are candidates for molecular targets for the treatment of diabetic nephropathy.
Recently, we have focused on elucidating the anti-inflammatory effects of modulators for nuclear receptors. We reported that peroxisome proliferator-activated receptor-γ (PPARγ) agnonist (Diabetes 2006), PPARδ agonist (Diabetes 2011), RXR antagonist (J Pathol 2012), and liver X receptors (LXR) agonist (J Am Soc Nephrol 2012) ameliorated the progression of diabetic nephropathy in rodent models by suppressing inflammatory pathways. We believe that the understanding of molecular pathways of inflammation could be translated into the development of anti-inflammation therapeutic strategies for diabetic nephropathy.
Reference:
・ Authors: Jun Wada, Hirofumi Makino
・ Title of original paper: Inflammation and the pathogenesis of diabetic nephropathy
・ Journal, volume, pages and year: Clin Sci (Lond) 2013 Feb 1;124(3):139-152.
・ Digital Object Identifier (DOI): 10.1042/CS20120198
・ Affiliations: Department of Medicine and Clinical Science, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.