Enlarge Image Figure 2. Synthesis of 9-Silabifuluorenes
Enlarge Image Figure 3. Asmmetric Synthesis of Chiral Spirosilabifluorene 2
Enlarge Image
Construction of silafluorenes based on transition metal catalyzed C-H activation
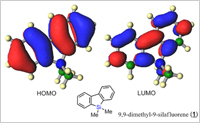
Siloles with two annulated benzo groups are referred to as silafluorenes or dibenzosiloles. Silafluorene derivatives have important industrial applications including photovoltaic devices/solar cells, and light-emitting diodes (LEDs), and as field effect transistors (FETs).
The conventional method for the synthesis of silafluorenes (Figure 1) consists of the lithiation of o,o'-dihalobiphenyl followed by quenching with a dichlorosilane. In order to introduce substituents at desired positions, several methods using transition metal complexes have been reported recently.
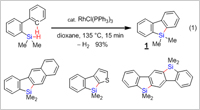
As new and novel strategy for the synthesis of silafluorenes, Takai and Kuninobu employed a method based on metal-catalyzed double activation of Si-H and C-H bonds with dehydrogenation. Tertiary silanes with a biaryl substituent were treated with Wilkinson's catalyst [RhCl(PPh3)3] for high yields of silafluorenes (Figure 2). The rhodium-catalyzed synthesis of silafluorenes from biphenylhydrosilanes via both Si-H and C-H bond activation is highly efficient because only dihydrogen is produced as a side product.
The advantage of this approach over other methods is that a silafluorene can be produced that contains a substituent or substituents on only one of the rings of the silafluorene. Furthermore, this method is also adaptable for the preparation of silafluorenes that contain other aromatic rings starting from suitably substituted phenylsilanes, and also for the construction of a ladder-type bis-silicon-bridged p-terphenyl compound (Figure 2).
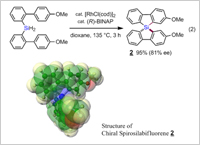
Examples of the synthesis of chiral organosilicon compounds are still rare among chiral organic compounds. This rhodium-catalyzed double dehydrogenative cyclization was employed for the construction of chiral bis(biphenyl)silanes having a quaternary silicon atom. Heating bis(biphenyl)silanes bearing substituents on the aromatic ring with a chiral rhodium catalyst gave chiral spirosilabifluorenes in high yields and enantiomeric excesses. The stereochemistry of the major enantiomer of the product was determined by X-ray single crystal structure analysis. Construction of chiral spirosilabifluorenes may lead to new research areas in materials chemistry (Figure 3).
Reference:
1.
・ Authors: Tomonari Ureshino,1 Takuya Yoshida,1 Yoichiro Kuninobu,1,2 and Kazuhiko Takai1
・ Title of original paper: Rhodium-Catalyzed Synthesis of Silafluorene Derivatives via Cleavage of Silicon Hydrogen and Carbon Hydrogen Bonds.
・ Journal, volume, pages and year: J. Am. Chem. Soc. 132 (41), 14324-14326 (2010).
・ Digital Object Identifier (DOI): 10.1021/ja107698p
・ Affiliations:
1: Division of Chemistry and Biotechnology, Graduate School of Natural Science and Technology, Okayama University, 3-1-1 Tsushima-naka, Kita-ku, Okayama, 700-8530 Japan
2: Present address: Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 Japan
2.
・ Authors: Yoichiro Kuninobu,1,2 Kanae Yamauchi,1 Naoya Tamura,1 Takayuki Seiki,1 and Kazuhiko Takai1
・ Title of original paper: Rhodium-Catalyzed Asymmetric Synthesis of Spirosilabifluorene Derivatives
・ Journal, volume, pages and year: Angew. Chem., Int. Ed. 52 (5), 1520-1522 (2013).
・ Digital Object Identifier (DOI): 10.1002/anie.201207723
・ Affiliations:
1: Division of Chemistry and Biotechnology, Graduate School of Natural Science and Technology, Okayama University, 3-1-1 Tsushima-naka, Kita-ku, Okayama, 700-8530 Japan
2: Present address: Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 Japan
・ Author website: http://www.achem.okayama-u.ac.jp/omc/index.html