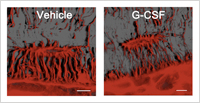
The projections of osteocytes are apparently suppressed by G-CSF administration.
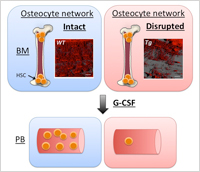
Enlarge Image While HSCs were mobilized from bone marrow (BM) to the peripheral blood (PB) by G-CSF administration in mice whose osteocyte network was intact (Blue panels), mobilization of HSC was severely impaired in osteocyte network disrupted mice (Red panels).
Enlarge Image
Osteocyte: Shadow commander of HSC niche
Hematopoietic stem/progenitor cells (HSPCs) reside in their niche in the bone marrow, which is the local environment that regulates stem cell fate including their trafficking. Clinically, cytokine granulocyte-colony stimulating factor (G-CSF) is used to mobilize HSPCs from the bone marrow into peripheral circulation, and they are harvested as a source of stem cells for transplantation. The mechanism of this HSPCs egress by G-CSF has been vigorously investigated, but is still not fully understood.
Now, the research group of Dr. Noboru Asada, Professor Mitsune Tanimoto (both at Okayama University) and Dr. Yoshio Katayama (Kobe University) has found a new player of HSPC niche controlling cell—osteocyte—which is buried in the mineralized bone tissue. Osteocytes are the most abundant cells that exists in bone tissue, and recent research has revealed that osteocytes act as sensors of mechanical stress to the bone and actively control the balance of bone remodeling.
At first, the researchers found that osteocytes were suppressed by G-CSF treatment both morphologically and functionally. This suppression of osteocytes by G-CSF was mediated by the sympathetic nervous system, since osteocytes express the beta-2 adrenergic receptor and surgical sympathectomy diminished the suppression effect of G-CSF on osteocytes. Mice with targeted ablation of osteocytes or a disrupted osteocyte network have comparable numbers of HSPCs in the bone marrow but fail to mobilize HSPCs in response to G-CSF. In addition, osteocyte deletion had an effect not only on the osteoblastic niche but also other niche controlling cells such as bone marrow macrophage.
Taken together, these results indicate that the bone marrow/bone niche interface is critically controlled from the inside of the bone matrix and establishs an important physiological role for skeletal tissue in hematopoietic function.
Reference:
・ Authors: Noboru Asada1,2, Yoshio Katayama2,3, Mari Sato2, Kentaro Minagawa2, Kanako Wakahashi2, Hiroki Kawano2, Yuko Kawano2, Akiko Sada2, Kyoji Ikeda4, Toshimitsu Matsui2, and Mitsune Tanimoto1.
・ Title of original paper: Matrix-Embedded Osteocytes Regulate Mobilization of Hematopoietic Stem/Progenitor Cells
・ Journal, volume, pages and year: Cell Stem Cell 12, 737-747, 2013
・ Digital Object Identifier (DOI): 10.1016/j.stem.2013.05.001.
・ Journal website: http://dx.doi.org/10.1016/j.stem.2013.05.001
・ Affiliations
1 Hematology, Oncology and Respiratory Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences
2 Hematology, Department of Medicine, Kobe University Graduate School of Medicine
3 PRESTO, Japan Science and Technology Agency
4 Department of Bone and Joint Disease, National Center for Geriatrics and Gerontology (NCGG)
Author website(Japanese): http://ninai.med.okayama-u.ac.jp/